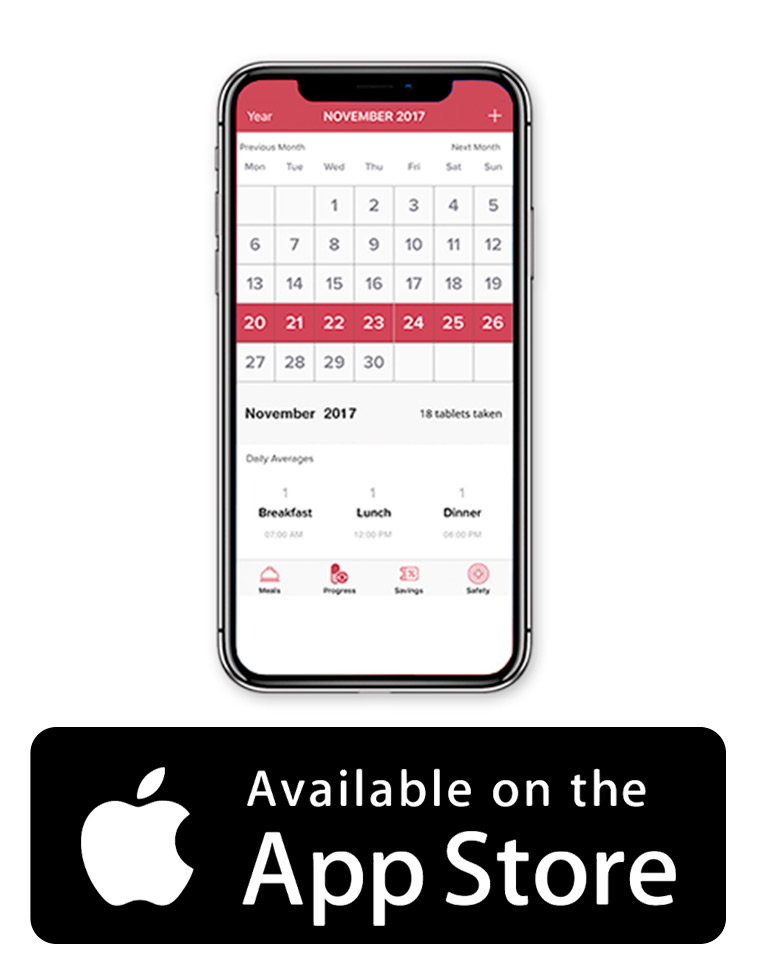
Lomaira Mobile App
Medications don’t work if they’re not taken. It is estimated that three out of four Americans do not take their medication as prescribed by the healthcare provider.1
The LOMAIRA Daily Dose Tracker app allows patients to enter the Lomaira daily dosing schedule and set an alert 30 minutes before each meal. In addition, the free app tracks a patient’s weight-reduction progress, provides refill reminders, and a visual history of LOMAIRA dosing.
Available for free on the Apple and Android app store

Lomaira Daily Dose Tracker
Lomaira app opening page.
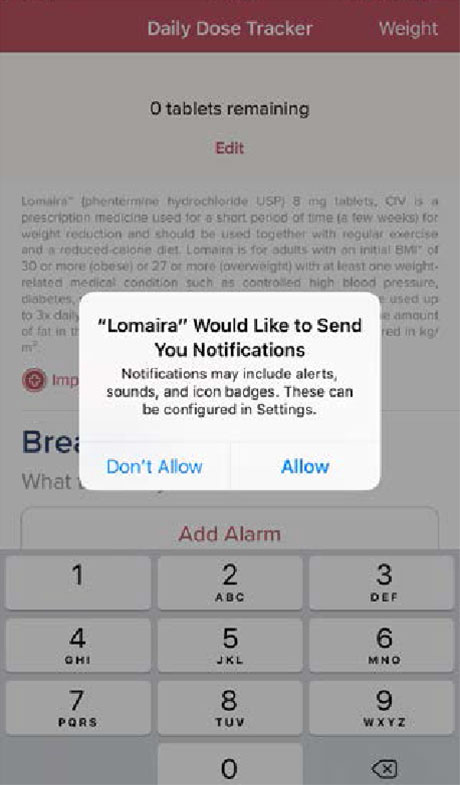
Step 1
Allow notifications so that the app can remind you to take your daily dose of Lomaira.
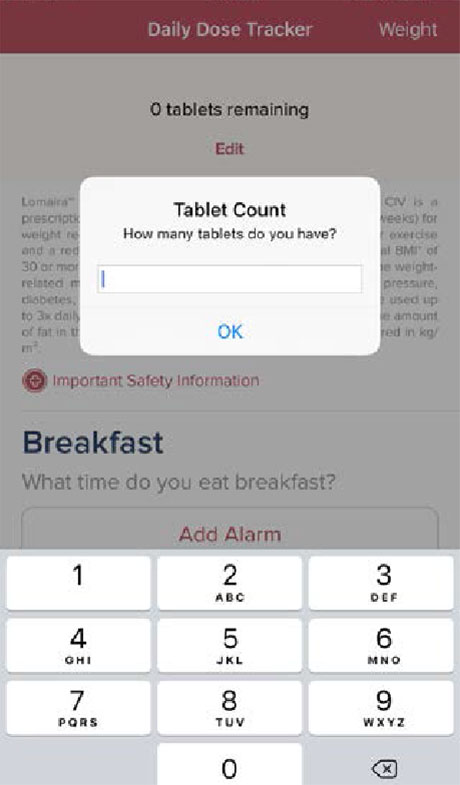
Step 2
Enter number of tablets prescribed by your healthcare provider. The Lomaira app will automatically track your medicine usage and alert you when it’s time to refill.
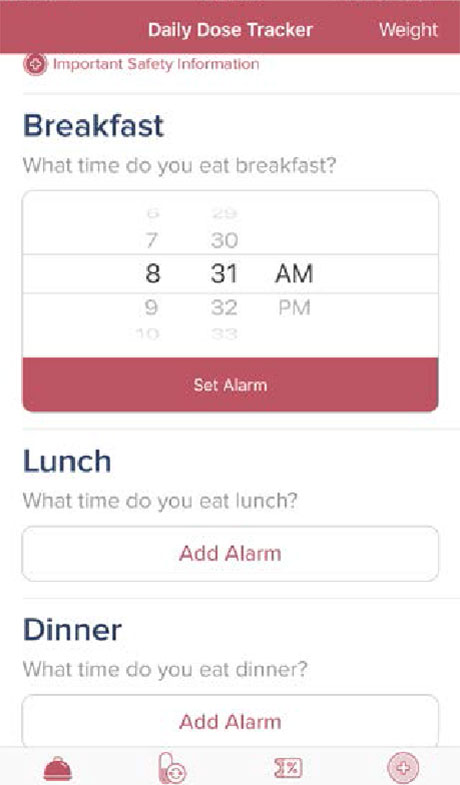
Step 3
Enter the typical time you eat breakfast, lunch, and dinner. You will be alerted to take your Lomaira tablet 30 minutes prior to your customized mealtime setting. Your digital calendar will sync automatically.
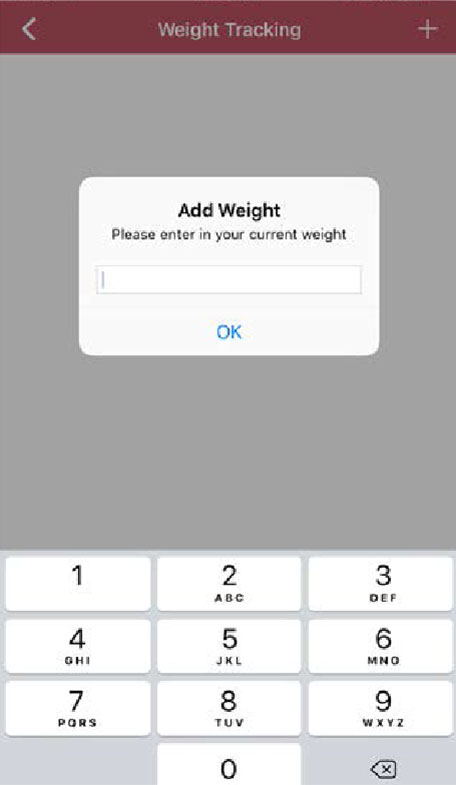
Step 4
Track your weightloss progress by clicking on “weight”, then the plus sign in the upper right corner and entering your current weight. You can enter your weight regularly by clicking on the “meals” icon in the lower left of the screen.
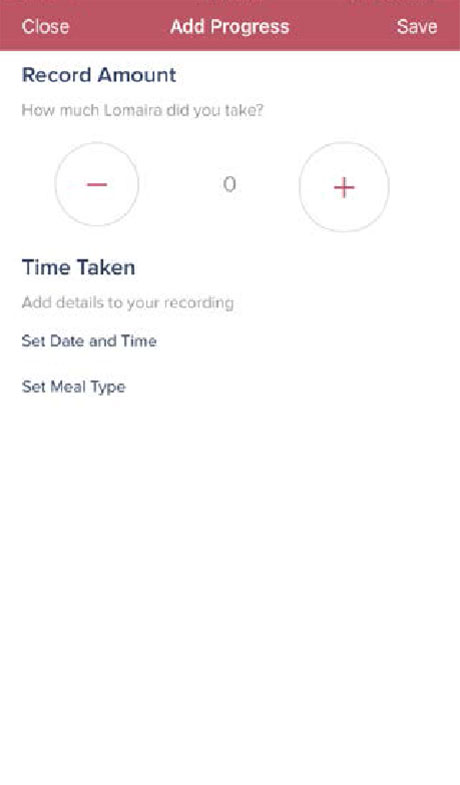
Step 5
Click the “Progress” icon, then the plus sign to enter the number of tablets, date and time, and meal type for your daily Lomaira dose. Click “Save”.
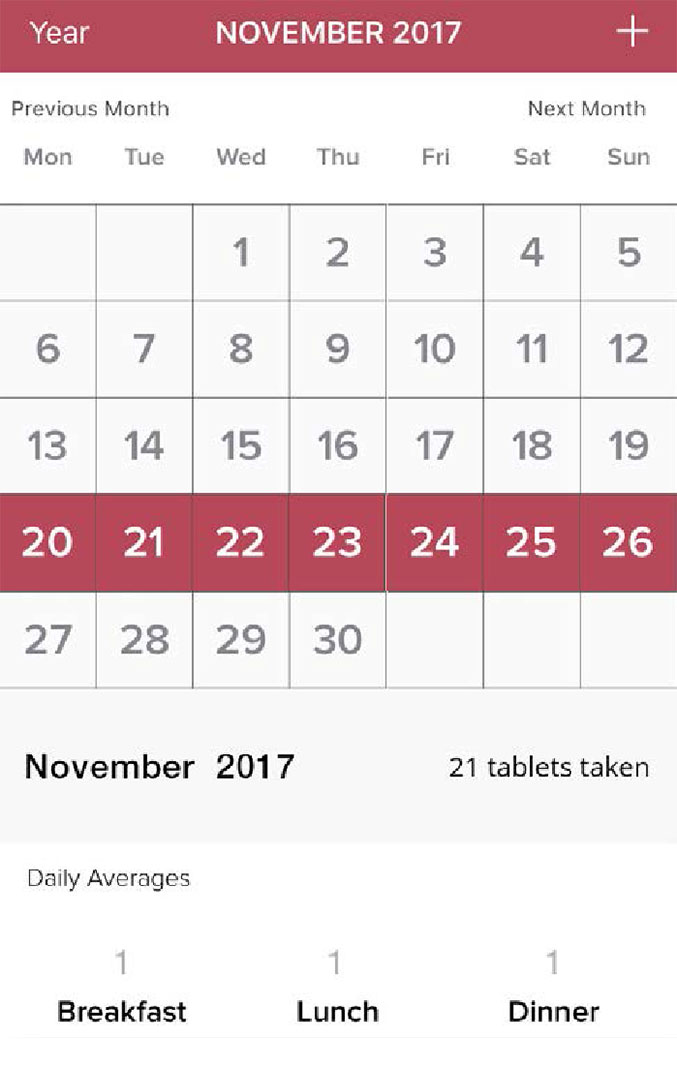
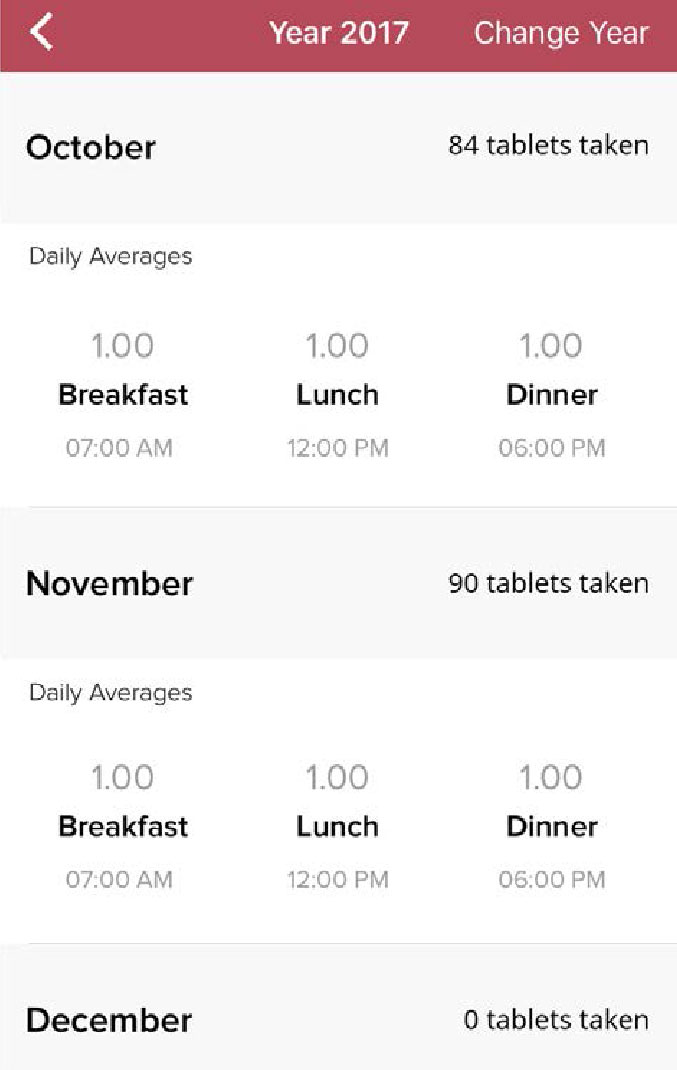
Step 6
View the history of your Lomaira treatment and dose adjustments by clicking “Year” on the progress page.
Easy "How-to" instructions to set up your Lomaira app.
INDICATION
Lomaira™ (phentermine hydrochloride USP) 8 mg tablets, CIV is a prescription medicine used for a short period of time (a few weeks) for weight reduction and should be used together with regular exercise and a reduced-calorie diet. Lomaira is for adults with an initial BMI* of 30 or more (obese) or 27 or more (overweight) with at least one weight-related medical condition such as controlled high blood pressure, diabetes, or high cholesterol. The limited usefulness of this drug class (anorectics), including Lomaira, should be measured against possible risk factors inherent in their use.
IMPORTANT SAFETY INFORMATION
Don’t take Lomaira™ if you have a history of cardiovascular disease (e.g., coronary artery disease, stroke, arrhythmias, congestive heart failure or uncontrolled high blood pressure); are taking or have taken a monoamine oxidase inhibitor drug (MAOI) within the past 14 days; have overactive thyroid, glaucoma (increased pressure in the eyes), agitation or a history of drug abuse; are pregnant, nursing, or allergic to the sympathomimetic amines such as phentermine or any of the ingredients in Lomaira.
Taking phentermine with other drugs for weight loss is not recommended. Primary pulmonary hypertension (PPH), a rare fatal lung disease, has been reported in patients who had taken a combination of phentermine and fenfluramine or dexfenfluramine for weight loss. The possible association between phentermine use alone and PPH cannot be ruled out. Patients should report immediately if they experience any decrease in the amount of exercise that they can normally tolerate, shortness of breath, chest or heart pain, fainting or swelling in the lower legs.
Serious heart valve problems or disease have been reported in patients taking a combination of phentermine and fenfluramine or dexfenfluramine for weight loss. The possible role of phentermine has not been established, therefore the possibility of an association between heart valve disease and the use of phentermine alone cannot be ruled out.
If your body becomes adjusted to the maximum dose of phentermine so that its effects are experienced less strongly, the maximum dose should not be exceeded in an attempt to increase the effect.
Caution is advised when engaging in potentially hazardous activity such as driving or operating machinery while taking phentermine. Phentermine has the potential to be abused. Keep Lomaira in a safe place to prevent theft, accidental overdose, misuse or abuse. Using alcohol with phentermine may result in an adverse drug reaction.
Phentermine can cause an increase in blood pressure. Tell your doctor if you have high blood pressure, even if it’s mild. If you are taking medicines for type 2 diabetes, your doctor may have to adjust these medicines while taking phentermine.
Some side effects of phentermine that have been reported include pulmonary hypertension, valvular heart disease, palpitations, increased heart rate or blood pressure, insomnia, restlessness, dry mouth, diarrhea, constipation and changes in sexual drive. These are not all of the potential side effects of phentermine. For more information, ask your doctor or pharmacist.
To report negative side effects of prescription drugs, contact FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
*Body Mass Index (BMI) measures the amount of fat in the body based on height and weight. BMI is measured in kg/m2.
 Talk to a Doctor Online
Talk to a Doctor Online



 Pay Less Than 50¢ Per Pill
Pay Less Than 50¢ Per Pill