Phentermine has been used consistently for medical weight management since it was first approved in 1959.
What Is Phentermine?
Phentermine is in the class of drugs called “anorectics”, also known as appetite suppressants, and has been used consistently for medical weight management since it was first approved in 1959. Phentermine is indicated for short term use (a few weeks) for patients with a initial BMI* of 30 or more (obese) or 27 or more (overweight) with at least one weight-related condition such as controlled high blood pressure, diabetes, or high cholesterol and should be used in combination with regular exercise and a reduced-calorie diet.
The exact mechanism(s) for this suppressant effect is/are unknown, although phentermine is thought to act by increasing the number of various neurotransmitters (norephinephrine, serotonin, dopamine and possibly others) in the spaces between nerve cells in the brain. When these chemicals are increased, the feeling of hunger is minimized.
Phentermine continues to be the most commonly prescribed medicine for weight loss in the United States.
Recent FDA Actions
In recent years, the Food and Drug Administration (FDA) has approved five new prescription medicines to help adults manage their weight in combination with diet, exercise, and behavior modification. These medicines include Qsymia® (phentermine and topiramate extended-release) capsules CIV, Belviq® (lorcaserin HCl) CIV, Contrave® (naltrexone HCl and bupropion HCl) extended-release tablets and Saxenda® (liraglutide) injection. Despite the new entrants, phentermine continues to be the most commonly prescribed medicine for weight loss in the United States. Over 7 million prescriptions are written for phentermine yearly by a wide range of healthcare professionals (HCPs), including obesity medicine specialists, family practitioners, endocrinologists, internists and cardiologists.
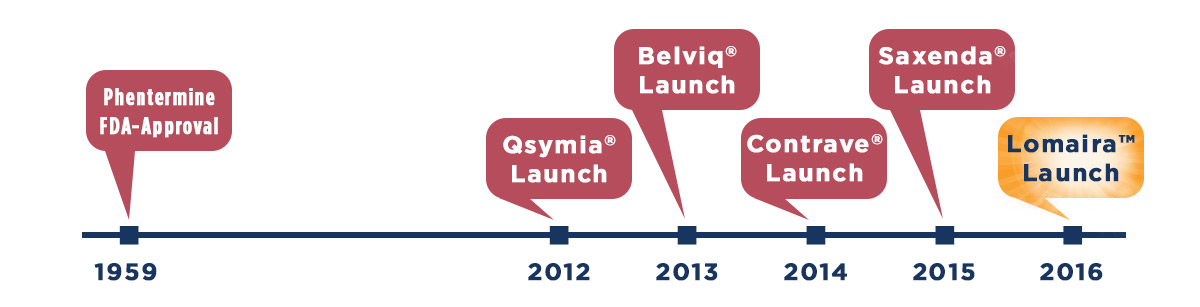
The fifth and most recent approval (September 2016) in the battle against the disease of obesity is Lomaira™ (phentermine hydrochloride USP) 8 mg tablets CIV, a low-dose formulation of phentermine that can be used up to three times a day before meals. Unlike current dosage forms of phentermine HCl [Adipex-P® (phentermine hydrochloride USP) CIV and generic phentermine 15 mg, 30 mg, and 37.5 mg] that are dosed in the morning before or shortly after breakfast, Lomaira can be dosed up to three times a day to target eating patterns throughout the day.
Lomaira can be dosed up to three times a day to target eating patterns throughout the day
Individualized Treatment
Now phentermine has more flexibility in dosing, and healthcare professionals and patients can work together to personalize a comprehensive weight-loss treatment plan so that patients can take the dose they need, when they need it. Dosage can be individualized to obtain an adequate response with the lowest effective dose.
Dosage should be individualized to obtain an adequate response with the lowest effective dose. The usual adult dose is one tablet three times a day 30 minutes before meals. This tablet is scored to facilitate administering one half of the usual dosage for patients not requiring the full dose. Phentermine hydrochloride is not recommended for use in pediatric patients 16 years of age or younger. Late evening medication should be avoided because of the possibility of resulting insomnia.
KVK Tech is the leading manufacturer of phentermine for the American public
Generic Phentermine
KVK Tech is the leading manufacturer of phentermine for the American public and produces the most commonly prescribed 37.5 mg tablet of phentermine HCl. Generic phentermine HCl is prescribed in doses ranging from 15 to 37.5 mg and should be taken once a day before or within a few hours of breakfast. Lomaira is now the only strength of phentermine HCl that is FDA-approved to be used up to three times a day before meals. All KVK products are manufactured, packaged, and distributed by KVK in Newtown, Pennsylvania, U.S.A.
*Body Mass Index (BMI) measures the amount of fat in the body based on height and weight. BMI is measured in kg/m2.
Adidpex-P® is a registered trademark of Teva Pharmaceuticals USA, Inc. Belviq® is a registered trademark of Arena Pharmaceuticals GmbH. Contrave® is a registered trademark of Orexigen Therapeutics, Inc. Saxenda® is a registered tradedmark of Novo Nordisk, Inc.
INDICATION
Lomaira™ (phentermine hydrochloride USP) 8 mg tablets, CIV is a prescription medicine used for a short period of time (a few weeks) for weight reduction and should be used together with regular exercise and a reduced-calorie diet. Lomaira is for adults with an initial BMI* of 30 or more (obese) or 27 or more (overweight) with at least one weight-related medical condition such as controlled high blood pressure, diabetes, or high cholesterol. The limited usefulness of this drug class (anorectics), including Lomaira, should be measured against possible risk factors inherent in their use.
IMPORTANT SAFETY INFORMATION
Don’t take Lomaira™ if you have a history of cardiovascular disease (e.g., coronary artery disease, stroke, arrhythmias, congestive heart failure or uncontrolled high blood pressure); are taking or have taken a monoamine oxidase inhibitor drug (MAOI) within the past 14 days; have overactive thyroid, glaucoma (increased pressure in the eyes), agitation or a history of drug abuse; are pregnant, nursing, or allergic to the sympathomimetic amines such as phentermine or any of the ingredients in Lomaira.
Taking phentermine with other drugs for weight loss is not recommended. Primary pulmonary hypertension (PPH), a rare fatal lung disease, has been reported in patients who had taken a combination of phentermine and fenfluramine or dexfenfluramine for weight loss. The possible association between phentermine use alone and PPH cannot be ruled out. Patients should report immediately if they experience any decrease in the amount of exercise that they can normally tolerate, shortness of breath, chest or heart pain, fainting or swelling in the lower legs.
Serious heart valve problems or disease have been reported in patients taking a combination of phentermine and fenfluramine or dexfenfluramine for weight loss. The possible role of phentermine has not been established, therefore the possibility of an association between heart valve disease and the use of phentermine alone cannot be ruled out.
If your body becomes adjusted to the maximum dose of phentermine so that its effects are experienced less strongly, the maximum dose should not be exceeded in an attempt to increase the effect.
Caution is advised when engaging in potentially hazardous activity such as driving or operating machinery while taking phentermine. Phentermine has the potential to be abused. Keep Lomaira in a safe place to prevent theft, accidental overdose, misuse or abuse. Using alcohol with phentermine may result in an adverse drug reaction.
Phentermine can cause an increase in blood pressure. Tell your doctor if you have high blood pressure, even if it’s mild. If you are taking medicines for type 2 diabetes, your doctor may have to adjust these medicines while taking phentermine.
Some side effects of phentermine that have been reported include pulmonary hypertension, valvular heart disease, palpitations, increased heart rate or blood pressure, insomnia, restlessness, dry mouth, diarrhea, constipation and changes in sexual drive. These are not all of the potential side effects of phentermine. For more information, ask your doctor or pharmacist.
To report negative side effects of prescription drugs, contact FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
*Body Mass Index (BMI) measures the amount of fat in the body based on height and weight. BMI is measured in kg/m2.
 Talk to a Doctor Online
Talk to a Doctor Online



 Pay Less Than 50¢ Per Pill
Pay Less Than 50¢ Per Pill



